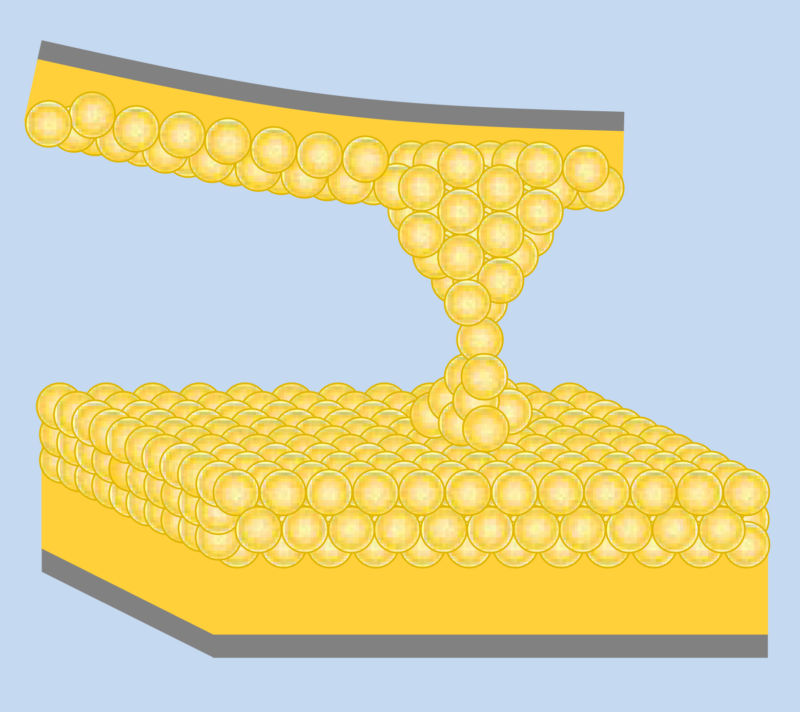
Enlarge / Heat at the bottom, a thermometer at the top, and just a single atom to bridge the two. (credit: University of Buffalo)
Thermal transport—the way heat is carried away from a processor, for instance—is very familiar to us. Viewing it as a quantum phenomena, by contrast, is quite alien. But heat is carried by electrons and phonons (phonons being the equivalent of photons for mechanical vibrations), and these are quantum objects. As a result, heat transport should be quantized into steps, just like electron conductance is. A recent paper shows that it’s a bit more complicated than that. Yes, the thermal conductance of materials varies in fixed steps, but that’s only true for some materials.
The thermal properties of bulk materials can be described as a combination of electrons and phonons that transport energy through a solid. This description means that heat transport should have some element of discreteness to it. Phonons and electrons can only take on the specific energy values that are allowed by their environment.
At high temperature, though, you should abandon all hope of seeing any discrete behavior. You can act as if the phonons and electrons can take on any energy (because the energy spacing is so small) and get accurate predictions. This approximation is how you end up with all the rules of heat conduction that engineers know and love.




